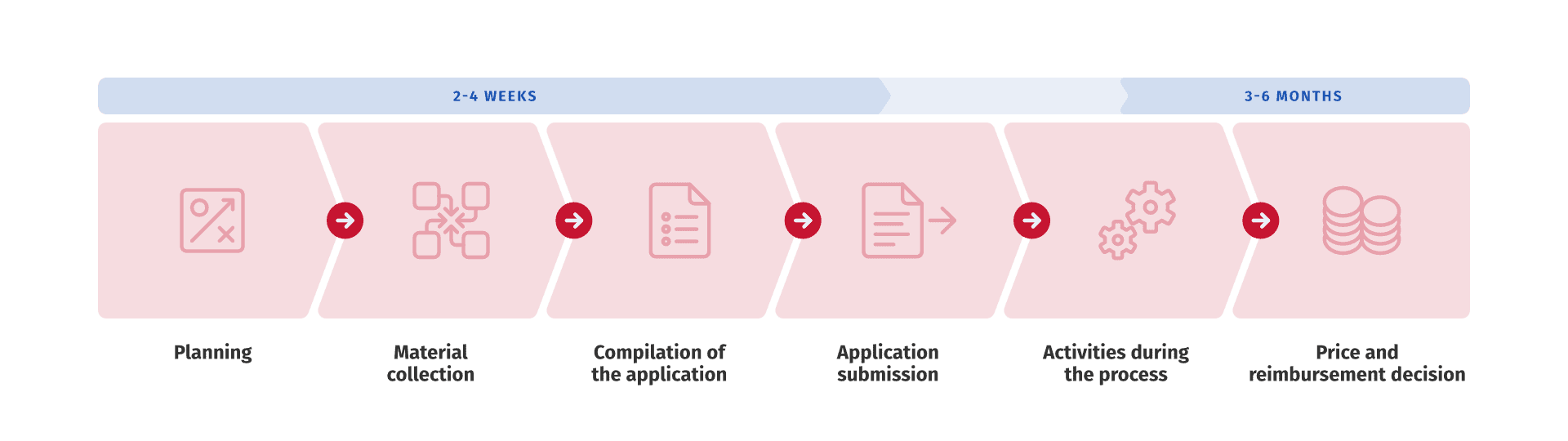
Sweden’s TLV Updates Reimbursement Model for Rare Diseases
Sweden has introduced a new reimbursement model for orphan drugs, likely to increase treatment options for rare diseases. At the same time, pharmaceutical companies are now expected to provide local evidence.