What happens to a patient in real life is visible through Real-World Evidence
A real-world evidence (RWE) study makes visible what happens to a group of patients in real life. The leap it takes away from the patient’s bed gives a broader perspective on what’s happening. Without RWE, the overall picture could remain unknown for those close to the patient and responsible for decisions.
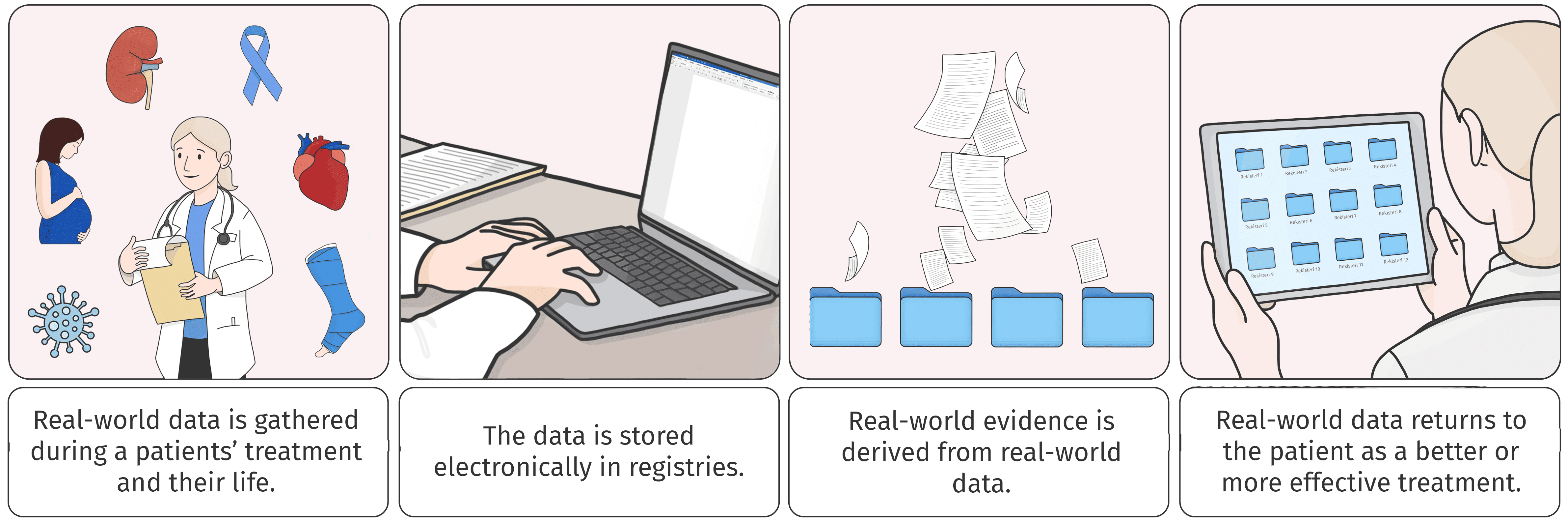
The comic strip above presents the journey of real-world data (RWD) from the patient’s bedside to decision-making and back to the patient’s care. Each time you finish the comic strip, you can begin reading it from the first panel. RWE is like a boomerang that returns to the patient’s side, improving the treatment or its efficiency.
It is vital to use registry data more comprehensively than for the purpose for which it was initially collected – for the so-called secondary purpose. This sort of research has the potential to turn today’s treatment into better treatment for future patients.
RWD is recorded electronically in health and social care registries during a patient’s treatment and life. The health information, such as the prescription, the reimbursement, and insurance information, and the data stored by the patient or their device are examples of RWD that the controller keeps.
RWD and its secondary use allow retrospective monitoring of a patient population and the related treatment and life events. Only a large amount of research subjects can enable significant examinations – RWD retrieved from a single person or a few persons would not create statistically significant RWE. Each participant is important, but individuals are no longer identifiable from the outcome of an RWE research.
”It would be fascinating to return to the time and place of recording a single patient data. However, this story would belong to a case report or medical drama television series, not the RWE study.”
In addition to demonstrating patients’ care and life events, RWD reflects the events at patient care on many levels. The hectic nature of patient care is sometimes visible in inaccuracies and errors in the recorded RWD – and no wonder as often both life and death are present at the time of recording! In an RWE study, recording errors are tackled with the assistance of a clinical expert and a sufficiently large dataset.
RWE is evidence obtained from RWD. RWE can be utilized for many purposes, such as to develop better service and care, more effective medicines, health-promoting, and supportive applications, and health technology.
Further thoughts
As the year is coming to a close and the next beginning, I still have intriguing blog post topics I would like to share with you. To name a few, you will be reading about RWE success stories and patient groups. The guest writer series world of methodologies will also continue. So, stay tuned.
I’d love to hear your thoughts on what you would like to read considering the RWE topic. Please feel free to write your comments in the comment section of the LinkedIn post related to this blogging.

